

Established safety profile

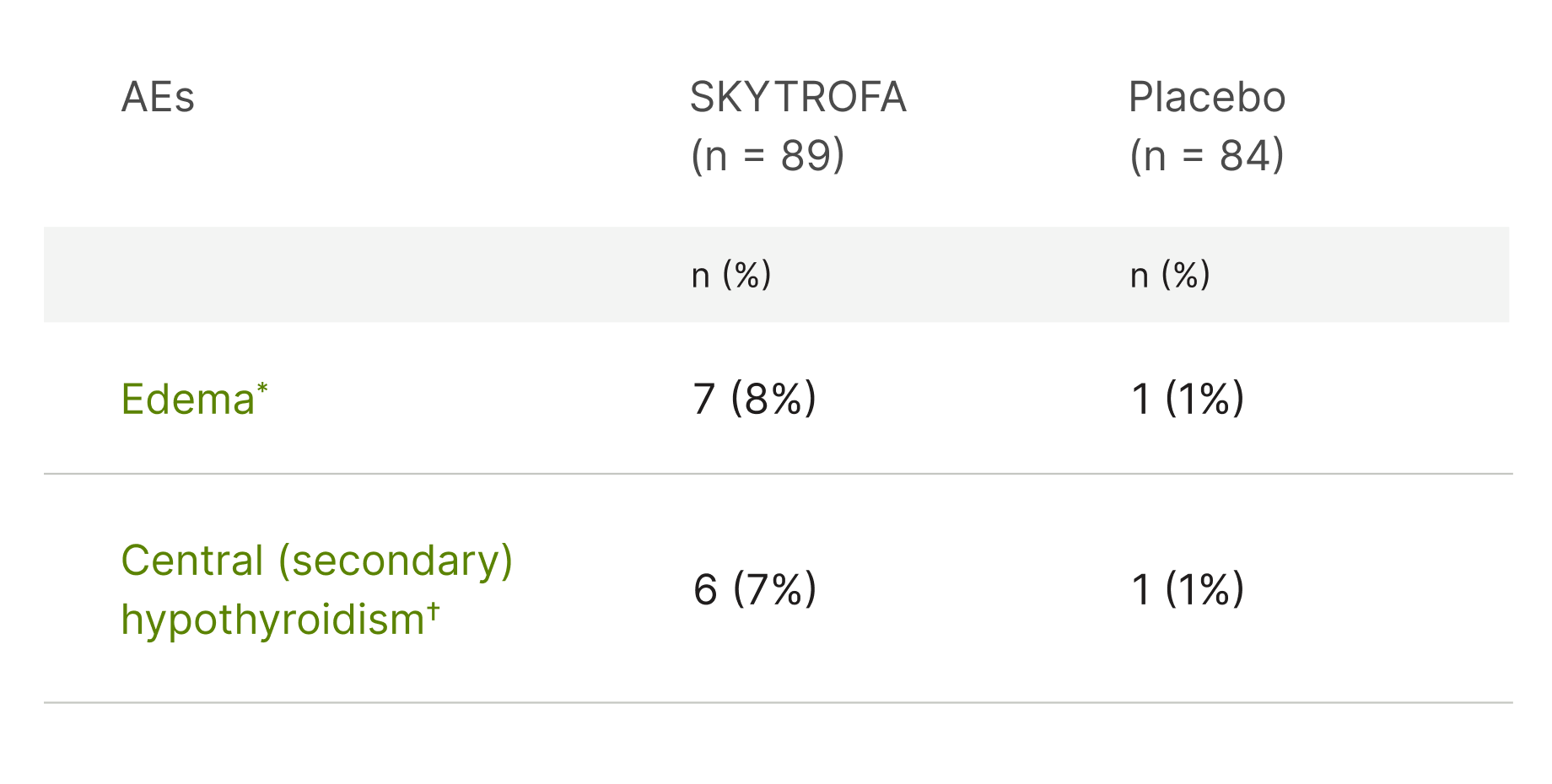
AEs occurring in ≥5% of SKYTROFA-treated adults and more frequently than in placebo-treated adults (38 weeks of treatment)1
- More SKYTROFA-treated patients shifted from normal or low baseline levels to elevated alkaline phosphatase levels at the end of the trial compared with the placebo group (14% versus 6%)1
- Low incidence of serious TEAEs in patients treated with SKYTROFA and placebo (4.5% and 1.2%, respectively)2
- The incidence of injection-site reaction was similarly low with SKYTROFA and placebo (4.5% and 4.8%, respectively)2
- AEs that are medically related were grouped in a single preferred term.
- *Edema in the SKYTROFA treatment group included edema peripheral (6) and peripheral swelling (1).
- †Central (secondary) hypothyroidism in the SKYTROFA treatment group included thyroxine free decreased (3), central hypothyroidism (2), thyroxine decreased (1), blood thyroid-stimulating hormone decreased (1), tri-iodothyronine free decreased (1). Preexisting central hypothyroidism in 5 of 6 SKYTROFA-treated patients.
Additional Safety Endpoints
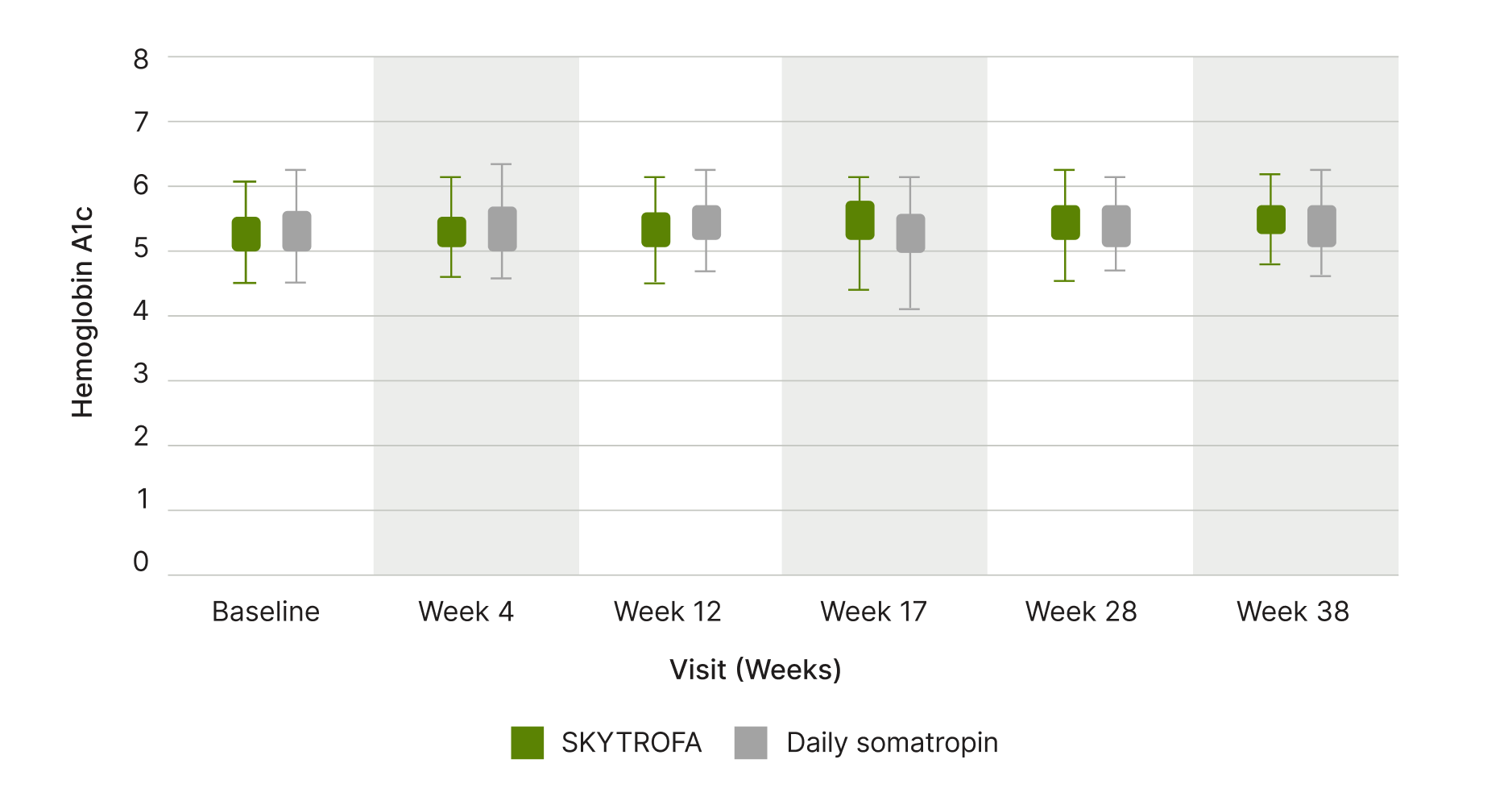
Impact on glucose metabolism
A1c remained stable with SKYTROFA and placebo3
Low immunogenicity
- Only 5.7% of adult patients with post-baseline assessments (14 of 247) had detectable antibodies against SKYTROFA1
- No clinically significant effect of antidrug antibodies on the safety and efficacy of SKYTROFA was observed1
- No neutralizing antibodies were detected1
- NOTE: New onset of impaired glucose tolerance/type 2 diabetes mellitus or exacerbation of preexisting diabetes mellitus can occur with somatropin treatment1
- Monitoring of blood glucose during treatment with SKYTROFA may be needed1
Study design

foresiGHt was a 38-week, multicenter, randomized, parallel-group, placebo-controlled (double-blind), phase 3 study with an additional active-controlled, open-label somatropin arm conducted in 259 adults with GHD: SKYTROFA (n = 89), placebo (n = 84), or daily somatropin (n = 86). The study enrolled patients aged 23 to 81 years who were GH treatment-naïve or had not received GH treatment during the prior 12 months. Doses were titrated over 12 weeks to reach the target maintenance dose by subgroup based on age and oral estrogen intake, with a fixed maintenance dose for the remaining 26 weeks of treatment. The primary efficacy endpoint was change from baseline in trunk percent fat compared with placebo, as measured by DXA, at week 38. The daily somatropin product arm (open-label) is included as a calibration arm to assist clinical judgment of the trial results. No formal statistical comparison between SKYTROFA and daily somatropin was conducted.1,2,4
foresiGHt study: Demographics and baseline characteristics2
| SKYTROFA | Placebo | Somatropin | |
|---|---|---|---|
| (n = 89) | (n = 84) | (n = 86) | |
| Age, mean (SD) | 43.0 (13.4) | 44.1 (14.7) | 41.3 (14.3) |
| > 60 years, n (%) | 12 (13.5) | 11 (13.1) | 11 (12.8) |
| Female, n (%) | 42 (47.2) | 39 (46.4) | 38 (44.2) |
| GHD onset | |||
| Adulthood, n (%) | 50 (56.2) | 46 (54.8) | 49 (57.0) |
| Childhood, n (%) | 39 (43.8) | 38 (45.2) | 37 (43.0) |
| BMI (kg/m2), mean (SD) | 27.0 (5.0) | 28.5 (6.5) | 28.6 (7.2) |
| IGF-1 SDS, mean (SD) | –2.6 (1.0) | –2.7 (1.2) | –2.8 (1.0) |
IMPORTANT SAFETY INFORMATION
INDICATIONS AND USAGE
SKYTROFA® (lonapegsomatropin-tcgd) injection is a human growth hormone (GH) indicated for the:
- Treatment of pediatric patients aged 1 year and older who weigh at least 11.5 kg and have growth failure due to inadequate secretion of endogenous GH
- Replacement of endogenous GH in adults with growth hormone deficiency (GHD)
CONTRAINDICATIONS
SKYTROFA is contraindicated in patients with:
- Acute critical illness after open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure due to risk of increased mortality with use of somatropin
- Hypersensitivity to somatropin or any of the excipients in SKYTROFA
- Pediatric patients with closed epiphyses
- Active malignancy
- Active proliferative or severe non-proliferative diabetic retinopathy
- Pediatric patients with Prader-Willi syndrome who are severely obese, have a history of upper airway obstruction or sleep apnea, or have severe respiratory impairment due to the risk of sudden death
WARNINGS AND PRECAUTIONS
- Increased Mortality in Patients with Acute Critical Illness: Increased mortality has been reported after treatment with somatropin in patients with acute critical illness due to complications following open-heart surgery, abdominal surgery, multiple accidental trauma, and in patients with acute respiratory failure
- Severe Hypersensitivity: Serious systemic hypersensitivity reactions including anaphylaxis and angioedema have been reported with post-marketing use of somatropin products, including SKYTROFA. Inform patients and/or caregivers that such reactions are possible and that prompt medical attention should be sought if an allergic reaction occurs
- Increased Risk of Neoplasms: There is an increased risk of malignancy progression with somatropin treatment in patients with active malignancy. Any preexisting malignancy should be inactive, and its treatment complete prior to instituting SKYTROFA. In childhood cancer survivors treated with radiation to the brain/head for their first neoplasm who developed subsequent GHD and were treated with somatropin, an increased risk of a second neoplasm has been reported. Children with certain rare genetic causes of short stature have an increased risk of developing malignancies and should be carefully monitored for development of neoplasms. Monitor patients with a history of GHD secondary to an intracranial neoplasm for progression/recurrence of the tumor. Monitor patients carefully for development of neoplasms and/or increased growth/potential malignant changes of preexisting nevi. Advise patients/caregivers to report changes in the appearance of preexisting nevi
- Glucose Intolerance and Diabetes Mellitus: Treatment with somatropin may decrease insulin sensitivity, particularly at higher doses. Previously undiagnosed impaired glucose tolerance and overt type 2 diabetes mellitus may be unmasked. Monitor glucose levels in all patients, especially those with risk factors for type 2 diabetes mellitus, such as obesity or a family history of type 2 diabetes mellitus. When initiating SKYTROFA, monitor patients with preexisting type 1 or type 2 diabetes mellitus or impaired glucose tolerance closely, and adjust the doses of antihyperglycemic drugs as needed
- Intracranial Hypertension: Intracranial hypertension (IH) with papilledema, visual changes, headache, nausea, and/or vomiting has been reported in a small number of patients treated with somatropin. Symptoms usually occurred within 8 weeks of the initiation of somatropin and resolved rapidly after cessation of therapy/reduction of the dose. Perform fundoscopic examination prior to initiation of treatment and periodically thereafter. If papilledema is observed, stop the treatment. If somatropin-induced IH is confirmed, restart SKYTROFA treatment at a lower dose after IH-associated signs and symptoms have resolved
- Fluid Retention: May occur during somatropin therapy. Clinical manifestations of fluid retention (eg, edema, arthralgia, myalgia, nerve compression syndromes including carpal tunnel syndrome/paresthesia) are usually transient and dose dependent
- Hypoadrenalism: Patients receiving somatropin therapy who have or are at risk for pituitary hormone deficiency(s) may be at risk for reduced serum cortisol levels and/or unmasking of central (secondary) hypoadrenalism. Patients treated with glucocorticoid replacement for previously diagnosed hypoadrenalism may require an increase in their maintenance/stress doses following initiation of SKYTROFA therapy. Monitor patients with known hypoadrenalism for reduced serum cortisol levels and/or need for glucocorticoid dose increases
- Hypothyroidism: Undiagnosed/untreated hypothyroidism may prevent an optimal response to SKYTROFA. Monitor thyroid function periodically as hypothyroidism may occur or worsen after initiation of SKYTROFA
- Slipped Capital Femoral Epiphysis in Pediatric Patients: Slipped capital femoral epiphysis may occur more frequently in patients undergoing rapid growth and may lead to osteonecrosis. Evaluate pediatric patients receiving SKYTROFA with the onset of a limp or complaints of persistent hip or knee pain for slipped capital femoral epiphysis and osteonecrosis, and manage accordingly
- Progression of Preexisting Scoliosis in Pediatric Patients: Monitor patients with a history of scoliosis for disease progression
- Pancreatitis: Cases of pancreatitis have been reported in pediatric patients receiving somatropin. The risk may be greater in pediatric patients than in adults. Consider pancreatitis in patients with persistent severe abdominal pain
- Lipoatrophy: Lipoatrophy may result when somatropin is administered at the same site over a long period of time. Rotate injection sites to reduce this risk
- Sudden Death in Pediatric Patients With Prader-Willi Syndrome: There have been reports of fatalities after initiating therapy with somatropin in pediatric patients with Prader-Willi syndrome who had one or more of the following risk factors: severe obesity, history of upper airway obstruction or sleep apnea, or unidentified respiratory infection. Male patients with one or more of these factors may be at greater risk than female patients. SKYTROFA is not indicated for the treatment of pediatric patients who have growth failure due to genetically confirmed Prader-Willi syndrome
- Laboratory Tests: Serum levels of alkaline phosphatase and phosphate may increase after SKYTROFA therapy. Serum levels of parathyroid hormone may increase after somatropin treatment. If a patient is found to have abnormal laboratory tests, monitor as appropriate
ADVERSE REACTIONS
- Pediatric patients with GHD: the most common adverse reactions (≥ 5%) in patients treated with SKYTROFA and more frequently than in those treated with daily somatropin were viral infection, pyrexia, cough, nausea and vomiting, hemorrhage, diarrhea, abdominal pain, and arthralgia and arthritis
- Adult patients with GHD: the most common adverse reaction (≥ 5%) in patients treated with SKYTROFA and more frequently than in those treated with placebo were edema and central (secondary) hypothyroidism
DRUG INTERACTIONS
- Glucocorticoids: Patients treated with glucocorticoid replacement for hypoadrenalism may require an increase in their maintenance or stress doses following initiation of SKYTROFA
- Pharmacologic Glucocorticoid Therapy and Supraphysiologic Glucocorticoid Treatment: Adjust glucocorticoid dosing in pediatric patients to avoid both hypoadrenalism and an inhibitory effect on growth
- Cytochrome P450-Metabolized Drugs: SKYTROFA may alter the clearance. Monitor carefully if used with SKYTROFA
- Oral Estrogen: Patients receiving oral estrogen replacement may require higher SKYTROFA dosages
- Insulin and/or Other Antihyperglycemic Agents: Dose adjustment of insulin and/or antihyperglycemic agent may be required for patients with diabetes mellitus
You are encouraged to report side effects to FDA at (800) FDA-1088 or www.fda.gov/medwatch. You may also report side effects to Ascendis Pharma at 1-844-442-7236.
Please click here for SKYTROFA full Prescribing Information.
A1c = Glycated hemoglobin; AE = adverse events; BMI = body mass index; CI = confidence interval; DXA = dual-energy X-ray absorptiometry; GH = growth hormone; GHD = growth hormone deficiency; IGF-1 = insulin-like growth factor 1; LS = least square; SD = standard deviation; SDS = standard deviation score.
References: 1. SKYTROFA. Prescribing information. Ascendis Pharma Endocrinology, Inc.; 2025. 2. Gilis-Januszewska A, Biller BMK, Doknic M, et al. Results of the foresiGHt trial support the efficacy and safety of once-weekly lonapegsomatropin in adults with growth hormone deficiency (GHD). Abstract/poster presented at: Joint Congress of European Society for Paediatric Endocrinology and European Society of Endocrinology; May 10-13, 2025; Copenhagen, Denmark. 3. Ascendis Pharma Endocrinology, Inc. Data on file; 2024.
You are about to leave the Skytrofa.com website by opening a new webpage to a third-party website.
The site you are linking to is not controlled by Ascendis Pharma, and we are not responsible for the content presented on that site. Thank you for visiting Skytrofa.com.
US Healthcare Professionals
The information contained in this website is intended for US healthcare professionals only.
Please confirm that you are a US healthcare professional.
